Equipements


The confocal microscope is designed to acquire images with high spatial resolution cells or biological tissue by fluorescence. The principle uses a confocal pinhole (pinhole) adjusted to eliminate the fluorescence not coming from the focal plane. Lasers provide an intense light spot whitch is scanned on the preparation. The fluorescence emitted by each point is quantified and used to represent the brightness of the object (image).
This method provides a high resolution XY and XZ (gain up to 15% by XY and XZ 30% compared with an epifluorescence microscope)
- Stands reversed DMIRE2
- Lasers: Argon lines at 458, 476, 488, 514 nm (20mW)
Helium neon line at 543nm (1.5 MW)
Helium neon line at 633nm (10mW)
Important: Our system does not have UV ray. To view the nuclei you can use the TO PRO3, DRAQ 5 that will use the line 633 nm or propidium iodide that will use the 543nm line ...
- 3 Detectors for the fluorescence spectral coupled to a slot system (no emission filter)
- 1 Detector transmission / DIC
- Stage Motorized XY for acquisitions in multiposition and mosaics (accepts slide and 35mm Petri dish)
- Motorization Z via galvanometer stage
- Objectives: HCX PL APO CS 40X 1.25 oil (Rxy 156,2nm RXZ 334,4nm to 488nm) WD 100 .mu.m
HCX PL APO CS 63X 1.32 oil (Rxy RXZ 147.9nm 285.8nm to 488nm) WD 70 .mu.m
HCX PL APO CS 100X oil 1.4 (Rxy 139,4nm RXZ 235,8nm to 488nm) WD 90μm
This microscope provides an image with a xz resolution close to that obtained by a conventional confocal microscope. Scanning the sample is carried out by rapid rotation of a wheel consists of thousands of pinhole. When the wheel turns, the sample will be illuminated once per turn.
In the same way a conventional confocal microscope, only the fluorescence emitted from the focal plane will be collected by a CCD camera or sCMOS. The high speed of rotation of the wheel and an excitement to a relatively low level enables very fast acquisition while limiting photobleaching and phototoxicity.
- Stand reversed DMI4000
- Lasers: Laser 405nm UV ray (50mW)
- Blue Ray Laser 488nm (50mW)
- Cobolt Jive 561nm laser line (50mW)
- Coherent Laser Cube line 635nm (25mW)
- Semrock filters:
FF01 525/50
FF01 600 / 37-25
FF01 440/521/607/700
- Yokogawa spinning disk head CSU22
- High sensitivity EMCCD Camera Photometrics QuantEM 512SC
- Head FRAP with 2 405nm and 473 nm lasers for achieving photoactivation experiments and FRAP
- Objectives: HCX FL PLAN 10X 0,25 dry PH1 WD 5,9mm
HCX FL L 20X 0,4 dry PH1 WD 6,9mm
HCX FL PL FLUOTAR L 40X 0,6 dry PH2 WD 3,3mm
HCX PL APO 63X 1.4 oil PH3
HCX PL APO 100X 1.4 oil
- Motorized XY plate for acquisitions in multiposition (accepts blades and 35mm Petri dish)
- Temperature Control ensured by a temperature controlled room
- Controlled CO2 sample level
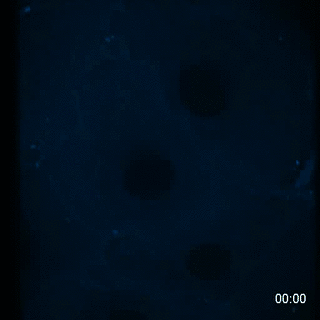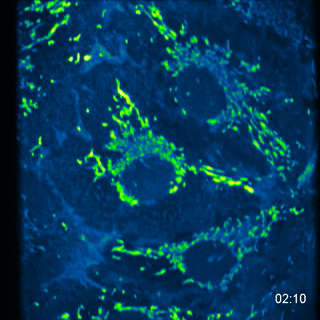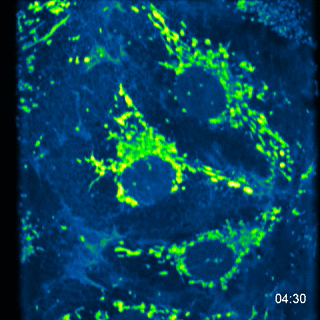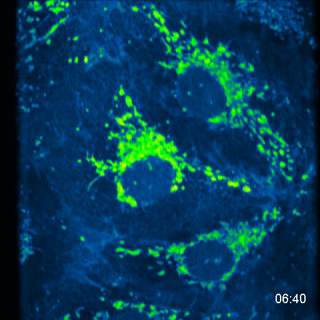
MA104 Cells- calcium probe MC164 - 5 images every 10s
The microscope field allows the acquisition of images and movies by a CCD camera, live or fixed specimens. Such samples may be small (cells, bacteria, yeast ...) or tissue sections.
Acquisitions may be made in three spatial dimensions (X, Y and Z), over time, in white light or multicolour fluorescence mode. Our microscope is equipped with a black and white camera (phase contrast, DIC, fluorescent) and a color camera (histo slide).
- Stand inverted Olympus IX83
- Black and white Camera: Hamamatsu CMOS sensor 4. Flash 2048x2048 pixels, pixel 6,5μm
- Color Camera: Olympus SC50. CMOS 2560x1920 pixels, pixel size 2,2μm
- Dichroic Filters Chroma:
"DAPI" excitement 350/50 emission 460/50
"EGFP" excitement 480/30 emission 535/40
"MCherry" excitement 560/40 emission 635/60
"Cy5" excitement 620/50 emission 690/50
- Light sources: LED or Xcite Xcite 120PC
- Software: Cell dimension sense v1.14 + Module Count and measure + deconvolution Module
- Objectives: UPLFLN 10X 0,3 dry WD 10mm
LUCPLFLN 20X 0,45 dry WD 6.6 to 7.8 mm correction collar
LUCPLFLN 40X 0.6 dry WD 6,27 to 4 correction collar
UPLSAPO 60XS2 Silicone WD 0.3 mm
- Platinum Motorized XY: possibility of multi-position, mosaic, blade scann
- CO2 Room: Pecon vivo cell
- Thermostatically Room: Pecon vivo cell

Time laps 24h phase contrast+fluo









